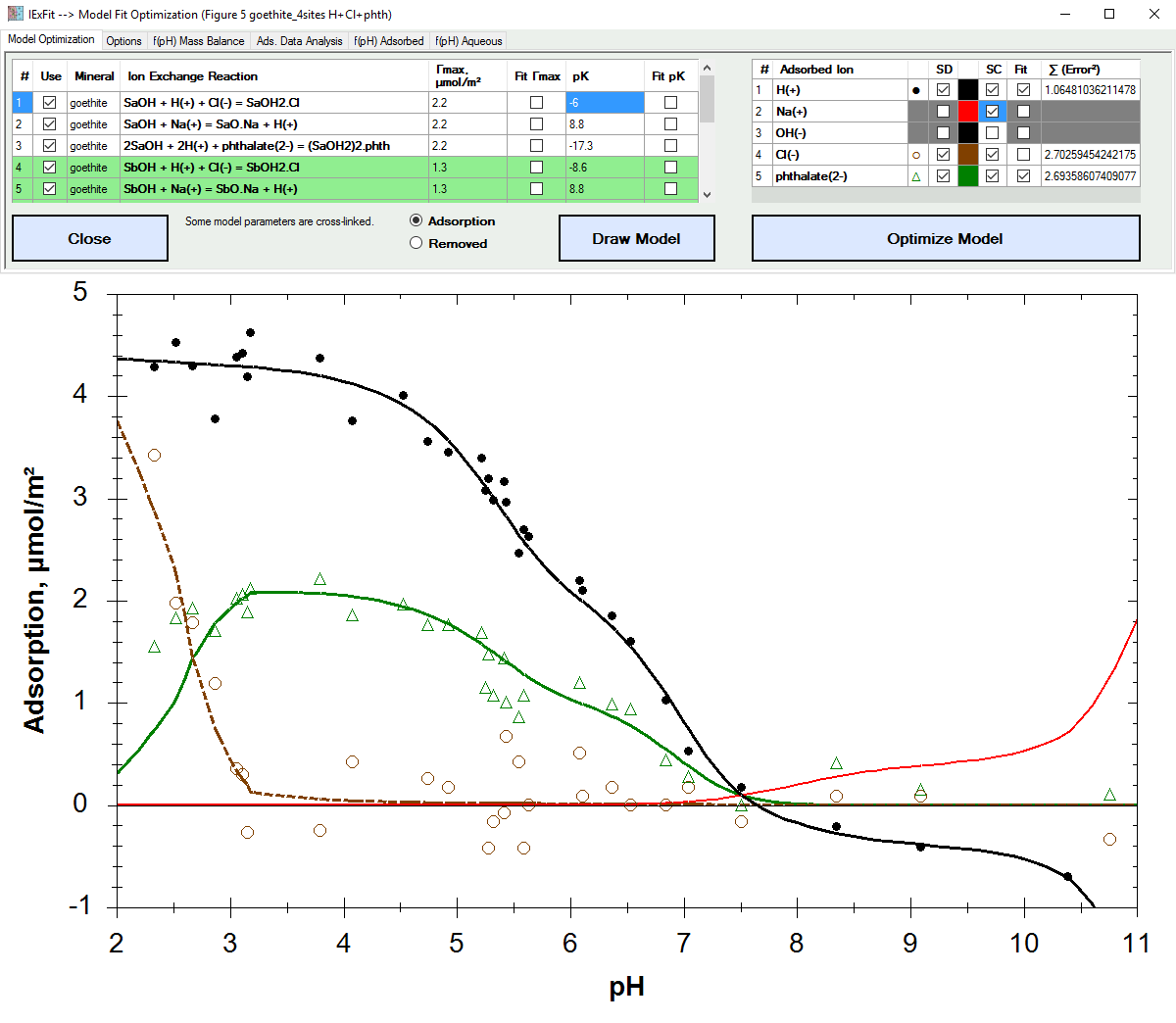
Fit adsorption data based on ion exchange reaction models.
Display solid & liquid & gas phase speciation diagrams as a funciton of pH & redox conditions.
| Alfisol, Item 007: | IExFit, version 3.4 Fit adsorption data based on ion exchange reaction models. Display solid & liquid & gas phase speciation diagrams as a funciton of pH & redox conditions. |
|
Software Overview |
|
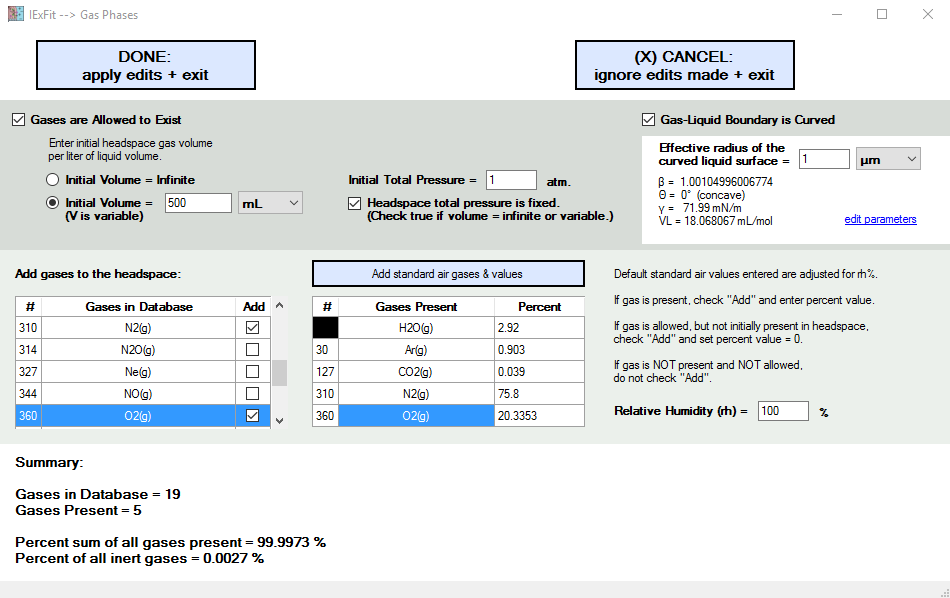
In the upper left corner here (see figure below), you decide if gases exist or are allowed to exist. If so, you also decide which ones and how much is initially present in the headspace per liter of liquid in your experiment.
The choices are:
(1) infinte volume (total pressure is fixed): select this to mimic a liquid with the whole atmosphere above it.
(2) initial volume is fixed (total pressure is variable): select this to mimic a closed bottle with a limited headspace above the liquid. That is, the bottle is closed with a cap.
(3) initial volume is variable (total pressure is fixed): select this to mimic a closed bottle with a variable headspace above the liquid. That is, the bottle is closed with an air bag (or balloon) at its mouth where a cap would normally be. If you are using a balloon, then presumably the balloon is large enough so as to never be filled to the point where it starts adding pressure when more gases enter the balloon. That is, the balloon is always limp, and the pressure inside the balloon equals the atmospheric pressure outside the balloon. The purpose of the balloon (or air bag) is only to avoid the internal air from mixing with the external air.
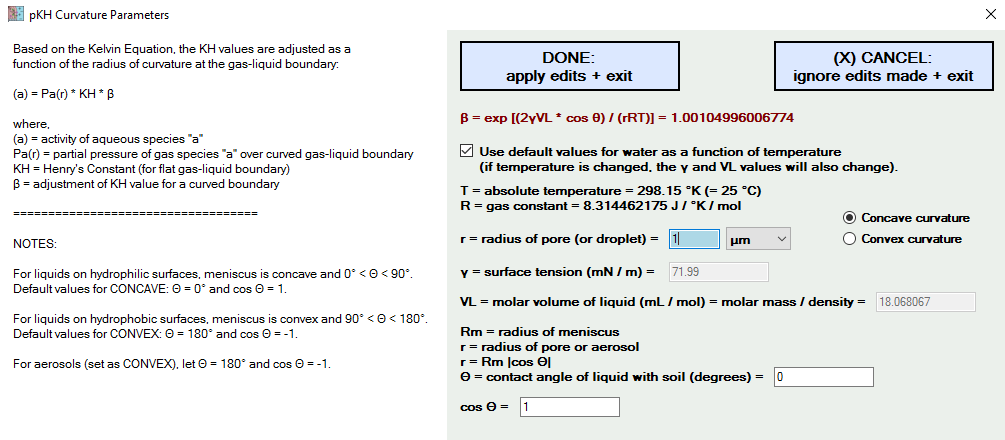
The important parameter of interest is the radius of curvature. Also, is it a concave or convex curvature. It is convex if its an aerosol (e.g., raindrop or mist) or a drop on a hydrophobic surface (e.g., some soils following a fire event). It is concave if it is in contact with a hydrophilic surface, as are most soils.